|
Combustion
Synthesis of Long Afterglow materials
Long
Afterglow: appropriate material to absorb high-energy radiation, and then they
emit light, the emitted photons whose energy is lower than the energy of
excitation radiation. This behavior has a light-emitting substance called
light-emitting substance. According to different excitation can be divided into
the photoluminescent material, cathode-ray-emitting material, an
electroluminescent material, a chemiluminescent material and so on. 1866 France
Sid ot finished first Z nS: Preparation of C u, the first to carry out the
research work in this series long afterglow luminescent material. Until the
early 20th century, long afterglow true realization of the industrial
production, also since then, has always been ZnS series dominated long afterglow
industry. To the 1990s, people began to find and focus on the long-lasting
materials with good performance and unique long-lasting light-emitting
properties of the rare earth doped, ushered in the maturing of super long
afterglow materials research and application of a new era. In recent years, rare
earth doped long afterglow materials have been widely used in areas such as the
instrument display panel concealed lighting and emergency lighting, aviation,
marine and automotive, it was also a rare earth doped long afterglow material
applied to the preparation of ceramics .
2,
light-emitting materials and long-lasting mechanism:
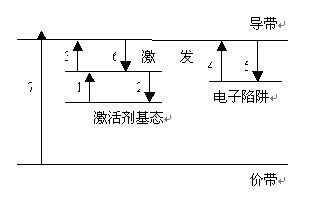
After stimulated luminescent material emitted discontinue called afterglow. Afterglow afterglow duration called afterglow time of less than 1 μs called ultrashort afterglow, called short afterglow, known in short afterglow 10μs-1ms between 1-10μs room, called 1-100ms between the afterglow called 100ms-1s between long-lasting, more than 1s called the super-long afterglow.The figure shows the level trap model can be used to explain the mechanism of long afterglow luminescent material, the specific mechanism is as follows: After the activator (donor) is incorporated into the matrix, near the conduction band in the band gap can be formed at a position a series of impurities level, to play a role in the conduction band trap movement of electrons, may be stuck in the trap for a long time, only in the external force will not be released; in the excitation photon, electron transitions from the ground state to the excited activator state ( Process 1); if the electronic direct return to the ground state energy which produce transient luminous phenomena (Process 2), is the fluorescence emission; light excitation will make some electronic transitions to the conduction band (process 3), and confined in the trap (process 4); If the energy level in the trap electrons get enough energy E, they will be released from the trap out (process 5), which is that they may be re-captured by the trap, it may be activated by the transition to the conduction band Agent ground state (process 6), and the light-emitting center complex, causing long afterglow luminescence that is.
The length of afterglow electron captured by the trap number N and the energy of these electrons gain of E related to: N, the more long afterglow time; E in a certain range, the more long afterglow time, but can reach the trap the value of the electronic release of all is that it will not help to prolong the afterglow time ......
Long-lasting materials are studied first luminescent material. Luminescent material is used as the main compound and a specific amount of impurity ions of the material and other components, the activator is a luminescence center, when it receives external excitation energy to produce light. Divalent europium ion activated aluminate phosphor, is a promising phosphor material. When it is subjected to blue light, the excitation light and nearly purple violet light, because of Eu2 + luminescence is f6d5-4f7 broadband allowed transitions, and 5d electrons in the outside world unshielded bare state, greatly influenced by the crystal field, so its luminescent characteristics and its chemical composition not only bright, but also bright and preparation.
3. A long-lasting synthetic materials
1, high-temperature solid-state reaction method
High temperature solid state reaction process is prepared by long-lasting: the molar ratio calculated in accordance spectrum weighed Grade A120, sr-co: and Eu203 raw materials, and the addition of a certain amount of flux after mixing ground, first by 1500 ℃ Sichuan burning three hours, and then the reducing gas and a 1400 ℃ 1200 ℃ baking 2-4 hours before to obtain greater hardness phosphor, crushed into small pieces, and then milled aluminum powder was prepared by acid-ming shop Changyu material. Due to high temperature solid state reaction synthesis of phosphors, slow crystal growth process requires a few hours at a high temperature, so the phosphor grains are gradually growing synthesis, coarse grains; furthermore synthetic phosphor hardness , after tests showed that it is harder than glass, between hardness 6-7. In order to put the system into a powder long afterglow phosphor material, it must be milling process. To do this, first remove the bulk phosphor broken into a certain size, and then use high hardness, high prices of corundum ball machine pulverized long-lasting material. Since the mill feed tank block size difference between the larger goal, in the milling, it is often just a large gob ground, while smaller blocks have been finely ground material, and it could undermine the long-lasting material crystal shape impact of brightness, and even make the emission luminance drop dramatically. According to Ming homemade aluminate phosphor shop pick imported from Japan's BM-3 comparison shows the measured luminance meter, ground powdered long-lasting luminescence brightness is only about a quarter of a block phosphor. Afterglow emission luminance powdery material decreases significantly, in addition to the main reason, there are finely ground raw material mixing problems. Because people rely on or after mechanical grinding and mixing, and then use high-temperature solid-state reaction method (dry method), it is impossible to achieve a very uniform degree, which can be observed on the phosphor prepared this section does not emit light points the formation of these points is not reached due to raw material grinding and mixing caused. To do this, we can match the solution by a certain percentage of raw materials, and then dehydrated avoid the same high temperature solid state reaction method (wet) preparation of long-lasting materials. As long as the process is reasonable, it can solve the traditional manual or milling the problem of uneven mixing.
2. The sol-gel method
Sol-gel method is a method of preparing a material which is beginning to prepare inorganic materials instead of conventional solid-state reaction method. Sol-gel method starting in the mid-nineteenth century, but until 1971 the German scholars use So-Gel method successfully prepared multi-component glass later, is now widely used in the preparation of ceramic materials and ultrafine materials t6J, etc., In recent years, gradually use 501 Gel prepared a phosphor. Sol-gel process is first prepared material preparation readily hydrolyzed metal soluble salt, and then after the hydrolysis and condensation processes in aqueous ethanol gradually gelled, in order to accelerate the rate of condensation reaction catalyst should be added, and finally through dehydration and from alcohol, drying and sintering process, only to obtain the desired material. Can be prepared by the sol-gel CasiO: Mn2 +, Pb2 +, phosphor materials were of analytical grade Ca (N03) 2 · 4 play O, Mn (CH3COO) 2 and pb (NO3) 2, and the spectrum of pure Si (OCH 3 ) 4, according to a certain ratio dissolved in aqueous ethanol, and incubated at 60 ℃ 3 a 4 hours, in order to strengthen a gel form, removing water and alcohol, so complex decomposition, the final heat treatment at 1150 ℃ 1 - 1.5 hours to obtain calcium silicate phosphor. Sol-gel method phosphor and high temperature solid state reaction method, the biggest advantage is the low synthesis temperature and prepared feed ingredient with highly uniform molecular level can be achieved uniformity, so the legal system SOl a fluorescent material Gel Optical better performance, but the content of activator required can be reduced. For the production of long-lasting materials, it can also reduce costs, enhance market competitiveness. Because the shop is part of a small content of rare earth resources in the ingredients, so production of small, high prices, the use of S01 Gel when a long-lasting material prepared can reduce spread content, will enable significant cost reduction.
3, combustion method
Method for preparing a powdered fluorescent material in addition to high temperature solid state reaction and sol-gel method, you can also use fluorescent materials prepared by combustion, we have successfully synthesized using combustion shop shop silver aluminate long-lasting materials, which It is at a lower oven temperature (500 ℃ a 700 ℃) conditions, fast (3 minutes to 5 minutes) synthesis of phosphors not agglomerate, small hardness, easily crushed, small grains, ground after the emission luminance drop was not significant . Preparation of the luminescent material combustion process is to first raw material nitrate, urea or glycine plus appropriate amount of reducing agent, thoroughly mixed after heating, when the reaction was exothermic reaction reaches ignition temperature, self-sustaining combustion after ignition combustion products is the desired fluorescent material. Rapid time by combustion synthesis of aluminate long-lasting material Abstract silver shop. Raw materials for the "fluorescent grade" EuZO3 and DyZO :, and after "analytical grade" SrC03 and Al (NO3) 3, according to a certain percentage of the previous three kinds of compounds dubbed nitrate solution, and the four kinds of raw materials in a particular mole fraction when mixed with the right amount of play Bo3 and urea, to be completely dissolved substances, it has been directly transferred into pre-heated to 600 ℃ in a muffle furnace. With the reduction of water, vessel solution was gradually showing a viscous sol state and continue to heat, can be observed as an oxidizer and a reducing agent urea nitrate dramatic exothermic oxidation-reduction reaction, to escape a lot of gas and then burning, burning flame exhibits reddish yellow light, the combustion process can be completed within tens of seconds, you can get a white "mushroom" shape product.
Prepared
by Combustion aluminate long-lasting material Abstract silver shop compared with
high temperature solid state reaction method, not only fast, energy-saving, and
products resulting from the combustion of pulverized into powder prepared
long-lasting material than the high-temperature solid-state reaction method were
powdered length afterglow material brightness, long afterglow time. This is
because the combustion is characterized by rapid movement of the combustion
crest, spontaneous high temperature (10000K-4000K), so long-lasting material
grains by the combustion synthesis of small, pulverized crystalline form after
almost not destroyed, the luminous brightness emission brightness difference
with the white "mushroom cloud" like product under the same
irradiation conditions were not significant. This sol-gel product prepared by
the same, crystal are small. However, the high temperature solid reaction
product prepared by the grains gradually grow and become, so coarse particles.
When grinding or milling,
There
are a large gob just grinding, while smaller slug has a finely ground. There may
even destroy the crystal shape, affect their luminescent properties, the
emission brightness significantly lower
Drop.
Preparation of the luminescent material combustion compared with the sol-gel method, can get small grains, excellent luminescent properties of the fluorescent material, but a sol-gel method silicate phosphor whole process takes four to 5.5 hours, longer than Combustion 4 a 5 minutes longer; and the sol-gel method requires a heat treatment at more than 10 degree heat, and the required combustion muffle furnace is only about 600 ℃, so the low temperature combustion process It is a big advantage. Compared with the high temperature combustion of solid state reaction and sol-gel method, obviously it has the advantage of saving time and energy, and to meet the energy requirements of green lighting material, so that the combustion process is the preparation of a promising new application of light-emitting material future Methods. In short, reasonable or preparation processes and methods, it is the key to good long-lasting material. Therefore, we should continue trying to find a more reasonable preparation methods, techniques and recipes like.
In
this study, long-lasting materials by combustion Preparation.
Experimental
part:
Purpose:
1,
learn quickly by combustion synthesis Afterglow material.
2,
to understand the principles of long-lasting materials and long-lasting.
3,
learn basic methods of inorganic oxides and nanomaterials Rapid combustion
synthesis.
Principle:
Combustion
method, also known as self-propagating high temperature synthesis method. Is a
highly exothermic chemical system to induce partial chemical energy by external
ignition, combustion wave form its frontier, so that the chemical reaction
continued to spread until the entire system response.
In
this study, through the use of metal nitrate and a mixed system of an organic
dye, which is formed by dissolving solution obtained after uniformly mixed,
high-temperature combustion synthesis aluminate, boiling, concentrated smoke
metal nitrates occurs at high temperatures in the flux, after the fire rapid
combustion, combustion flame in the form of spreading wave of self-sustaining
heaviest foamy powder.
2Al
(NO3) 3 + Sr (NO3) 2 + 20 / 3CO (NH2) 2 + 10O2 --- SrAl2O4 + 40 / 3H2O + 32 /
3N2 + 20 / 3CO2
Laboratory
equipment and materials:
Reagents:
Eu2O3, Dy2O3, strontium nitrate, aluminum nitrate, urea, boric acid, dilute
nitric acid
Equipment
and instruments: analytical balance, beakers, glass, pipettes, horse boiling
furnace, jade crucible fluorophotometer
Experimental
procedure:(A) to prepare a precursor: Quasi said 0.088 grams Eu2O3; 0.093 克 Dy2O3; 2.116 克 strontium nitrate, 7.5 g of aluminum nitrate, urea 15; 0.18 g of boric acid.1, with an amount of nitric acid was dissolved by heating to dissolve rare earth oxides, rare earth ions dubbed nitrate ion solution A.
2, weighed strontium nitrate, aluminum nitrate, urea, boric acid, with an amount of water dissolved with stirring in a state heated to about 70 ° C to give a solution B.3, under stirring, slowly added to B to A, and maintained with stirring while heating, and the system was uniformly mixed to evaporate some water.
4, the mixture was transferred to a crucible jade, i.e., the precursor can be obtained for combustion.
(B) the combustion of synthetic samples: first horse boiling furnace preheated to about 600 degrees Celsius, then the previous precursor fast into them, after a few minutes to observe the reaction was vigorously boiling, swelling, along with top-down spread , to be removed after the reaction, the product was cooled to obtain the synthetic foam - long-lasting material.
(C), characterized: after removing sample loading and emission spectra and spectrophotometer with the absorption spectrum of the sample, and the corresponding data, sorting through the fitting software will get relevant maps.
Third,
the results analysis
Fluorescence
excitation spectrum:
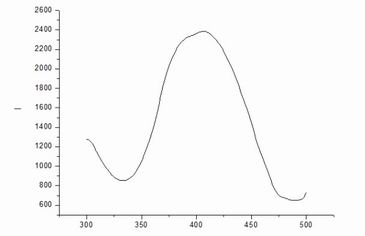
Data
and excitation spectroscopy: maximum intensity space corresponding excitation
wavelength, ie, the maximum excitation wavelength of 406.6nm, the wavelength
maximum punishment child absorbs energy, in the largest number of excited
states, which can produce the strongest fluorescence.
Fluorescent
emission light
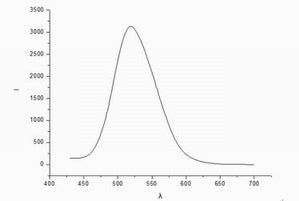
Data
and emission spectroscopy: the maximum intensity of the emitted light wavelength
corresponding to the premises, ie, the maximum emission wavelength of 518.6nm.
Summary,
due to the characteristics of the fluorescence measuring instrument, such as the
energy distribution of the light source, the sensitivity of the transmittance of
the monochromator and the detector are varied with wavelength, and thus
generally measured excitation and emission spectra, it is apparent spectrum.
Fluorescent light with a solution of the compound, the fluorescence measurements
on different instruments resulting spectra apparent, often differ from each
other only after the spectral shape correction is consistent.
|